Like many industries during the COVID-19 crisis, manufacturing has had to make big changes, including scaling back its workforce, enforcing new and intensified hygiene standards, and taking a closer look at workplace cleanliness. Looking ahead to the permanent adjustments we’re all making, it’s necessary to be aware of the ways new protocols could affect adhesion and cleaning processes in manufacturing.
In order to meet performance requirements for strong, reliable assemblies, manufacturers have needed to maintain and understand the cleanliness of material surfaces in their processes. At Brighton Science, we’ve always aimed to shed light on unusual or non-obvious variables that manufacturers may not be aware of or may not be looking at as possible conduits of contamination or leading the process toward failure. These inconspicuous variables can be changes made on the supplier side that cause unexpected issues downstream, cleaning and treatment steps that haven’t been fully optimized, or the transfer of contaminants from places that didn’t seem problematic.
One of these new variables introduced because of more robust hand washing and hand sanitizing conventions is the possibility of substances transferring from “clean” hands onto surfaces in a manufacturing environment. As companies begin bringing back their workforce, in order to ensure the safety and health of employees, there will be a higher degree of hygienic rules of conduct that will require more frequent hand washing as well as greater use of hand sanitizers and disinfectant wipes.
Increased use of personal cleanliness products raises questions about how easy it is for ingredients in these soaps and gels to transfer to critical surfaces in production. If they can transfer with any degree of ease, is their presence problematic to adhesion? Can surfaces still be considered clean enough for coating, sealing, painting, or printing operations if there are trace amounts of hand sanitizer on them?
Well, we wanted to find the answers ourselves, so we developed an experiment to see if we could find the data surrounding these queries.
Rethink your adhesion manufacturing processes with Surface Intelligence.
Cleanliness Matters
Before getting into the experiment itself, it’s useful to understand why these questions are even relevant to manufacturers. For any production process that includes adhesion of any kind, questions of cleanliness need to go beyond just what can be seen by the human eyes.
To learn more about how to fully control all of the variables in your adhesion and cleaning process to ensure predictable outcomes, download our eBook: Checklist: Adhesion Failure Root-Cause Analysis for Manufacturers.
In manufacturing, the cleaning and treatment of material surfaces exist to remove debris but also to create a surface that can be bonded to. The first half of that function (the removal of contaminants) is fairly straightforward. The second half (creating a surface that can be bonded to) requires control over the chemical changes that are occurring on the surface. Every wash, abrasion, activation, wipe, and treatment operation intentionally alters the chemical composition of the surface being interacted with. If this composition is altered in the proper way, adhesion performance can be optimized. Adhesion is a chemical reaction that occurs at the top few molecular layers of a surface.
Manufacturers have to exhibit control over the alteration of the molecules on the surfaces they’re cleaning in order to get the adhesion they require. This means if a chemical substance is introduced to a surface, they need to be certain it doesn’t interfere with adhesion or can be successfully cleaned off by the cleaning procedures already in place.
Hand Sanitizer and Soap Experiment
And that’s where our experiment comes into play. We began by flame-treating chrome-plated steel coupons or ferrotype plates that we would be subjected to unwashed, sanitized, and washed hands, respectively. In order to get a picture of what was on the surface of each sample, we used a surface analysis method called reflection absorption infrared spectroscopy (RAIRS) which can detect substances on surfaces as thin as a few molecules. Analysis done using RAIRS is expressed in spectra with peaks at certain wavelengths indicating the presence of particular substances.
We used two types of hand sanitizer (a gel and a foam) and two types of soap (also a gel and a foam). The ferrotype plates were first handled by unwashed hands to establish a baseline to compare the subsequently “clean” hands to. We also analyzed plates that were contaminated with a mixture of soap or sanitizer with an IPA solution that was sprayed onto the coupons. We wanted to get an understanding of what the soaps and sanitizers looked like on the surface when we knew they were present so we could be certain that substances transferred from our hands were, in fact, due to the soaps or sanitizers.
Human hands have skin oil that is typically composed of triglycerides and other fatty acids. The RAIRS results showed this with a prominent peak around 1600cm-1 (in the black box below), which is indicative of a carbonyl (C=O), showing the presence of fatty acids on the ferrotype surface.
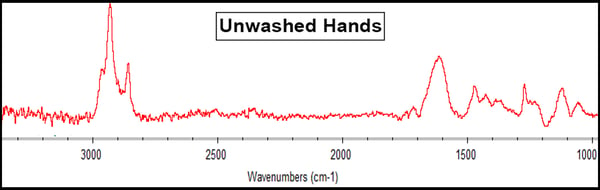
These hand oils can sometimes be harmful to bonding processes if they are not properly removed during the cleaning process. As always, what you need to do is contact Materials Science and Process Experts to map out and examine your specific process to understand the full extent to which these variables may affect your process. They’ll help you set up ways to measure the impact of contaminants and substances on your surfaces and measure how to clean your surfaces are before and after cleaning treatment and preparation processes.
For this experiment, in order to get as close to real-world results as possible, we applied one full press of the dispenser on top of the hand sanitizer bottle onto our hands and rubbed it in until dry before handling the ferrotype plates. Similarly, with the soaps, we used two full presses of the dispenser and followed guidelines that suggest vigorous hand washing for at least 20 seconds before rinsing. We then compared the spectrum above to a sample that had the sanitizers or soaps applied directly to the coupons and then the plates that were handled with the sanitized and/or washed hands.
One note to consider is that there was likely less time between hand washing or sanitizing and the subsequent handling of the samples than there would be in a real work scenario. Increased time before contact with the ferrotype plates could have affected the results, but these readings were taken within a few minutes of the hand cleaning. Additionally, increased contact time would likely result in a greater transfer of contaminants so the spectra we present here are likely from cleaner examples than there would be in a real-world situation.
The gel sanitizer offered these results:
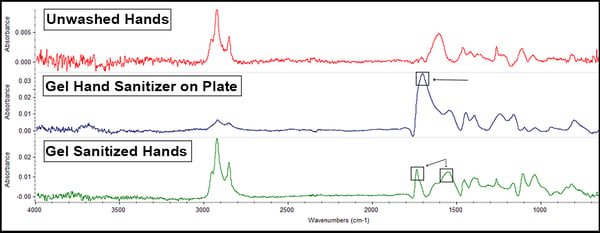
The biggest thing to note here is that the gel sanitizer possesses glyceryl caprylate, an ester that’s observable in the peak around 1700cm-1 (the box on the middle line). Since this peak, and the hand oils peak at 1600cm-1 (noted by the boxes on the bottom, green line) are still visible after handling indicates that not only is the sanitizer not removing the hand oils (which is not what sanitizer is supposed to do) but there is some transfer of the caprylate onto the coupon.
Hand sanitizer works the same way that antibacterial sprays and wipes work. Their purpose is to kill microbes that cause illness, and in order to do this, the gels or liquids need to stay on your skin or on a kitchen counter or office desk for several minutes to do their work thoroughly. There is no rinsing step with these, and while they are very good at killing disease-causing bacteria, they are intended to stay on your hands for a while after it is rubbed into your skin.
The foaming sanitizer offered these results:
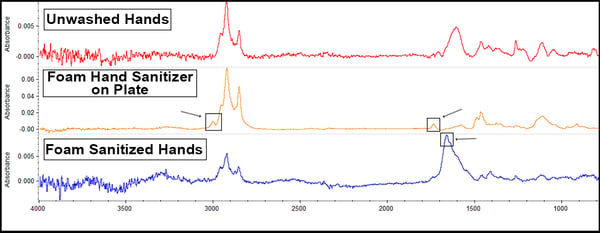
There are tiny peaks just above 3000cm-1 and at 1750cm-1 (on the middle yellow line) because the foam sanitizer possesses aromatic hydrocarbons, but these aren’t really apparent after being applied to the hands. However, there is a peak at around 1700-1600cm-1 (on the bottom line), suggesting possible reactions or material transfer happening with the sanitizer and the hands leading to hydrocarbon deposition onto the surface.
The gel soap offered these results:
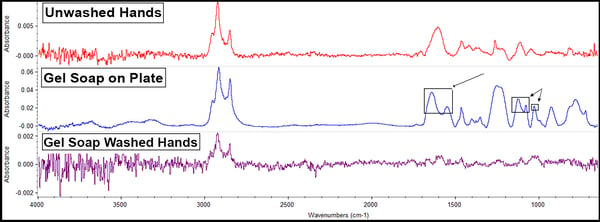
Soaps possess sulfates for disinfecting purposes; in this case, the gel soap contains sodium lauryl sulfate. These compounds have bands observable on the spectra at 1190-1160cm-1 and 1080-1015cm-1 (on the middle blue line). Soaps also possess fatty acid salts (a lathering agent) identified around 1650-1500cm-1 (also on the middle blue line). Note that no peaks are added to the sample handled with soap-washed hands (the bottom purple line). Only preexisting peaks indicating the presence of skin oils remain and at a lower amount. This indicates that rinsing with water and drying after washing hands removes essentially all of the soap, which is not only the recommended method of use on the bottle but is the way nearly everyone actually washes their hands.
The foaming soap offered these results:
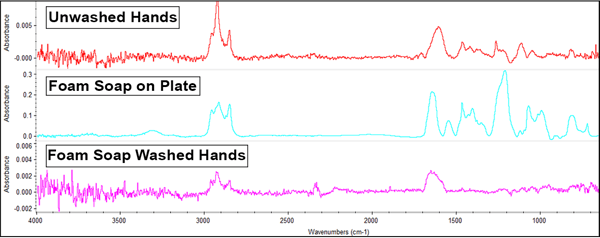
The foaming soap possesses similar prominent peaks (on the middle line) to the gel soap, indicating sulfate and fatty acid salt present in the reference spectrum. Also similar to its gelatinous counterpart, only existing peaks from the unwashed hand's sample are present in the washed sample, and to a lesser degree. Again this indicates that the soap is cleaning off many of the oils and hydrocarbons present on unwashed hands and does not transfer anything new to the samples.
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
Conclusions
The results are interesting but not necessarily surprising. Some of the personal cleaning agents were shown to transfer materials to the sample surfaces via passing touch. These contaminants (oils, sulfates, caprylates) could negatively impact adhesion if they are present in an incompatible material system. So, consult with Material Scientists to make absolutely sure these sorts of substances won’t interfere with your process or how to sufficiently clean them off if they could cause issues.
RAIRS showed that gel-based hand sanitizer, in particular, does little to detract from common skin oil, adding an additional layer of contaminant. The foaming sanitizer was less problematic, adding only a small amount of hydrocarbon to skin oil, but it still deposited more than what regular, old unwashed hands would. Soap and water decreased the presence of existing IR peaks supporting the well-accepted idea that hand washing is far more effective at removing contaminants that exist on our hands than sanitizer is since sanitizer isn’t actually removing anything without a rinse.
In order to preserve health and wellness, hand washing and hand sanitizing is essential and worth the extra time it may take to do properly. The transfer of some of the chemicals in the soaps and sanitizers onto surfaces is certainly possible but should not detract from the use of these throughout the day, every day. Much like all variables that come into play in manufacturing processes, these substances can be properly handled and removed from surfaces they don’t need to be on.
The key is to stay aware of the quality of your materials’ surfaces throughout the manufacturing process. Measure this quality with instrumentation that is sensitive to the chemicals at the top few molecular layers of the surfaces. Without this insight, you may never know if something transferred from a slight touch or not.
To learn more about how to fully control all of the variables in your adhesion and cleaning process to ensure predictable outcomes, download our eBook with a step-by-step guide to investigating the root causes of adhesion issues. If you can suss out where the problems stem from, then you can stop them before they wreak havoc. Get your copy today; checklist: Adhesion Failure Root-Cause Analysis for Manufacturers.


