One of our favorite services we provide to manufacturers is to help optimize surface preparation techniques for their particular materials and products. It’s our specialty and one of our biggest passions. We feel so strongly about it because we know that if you, as a manufacturer, are able to control the many variables in your production process that affect your adhesion outcomes, then you have a predictable adhesion process within your grasp. And that’s what we want to see for all manufacturers.
A study titled “Surface functionalization of automated fiber placement manufactured composites by atmospheric pressure plasma jet” was published in the International Journal of Adhesion and Adhesives. The methodology employed by authors utilized our method of process control (namely the Surface Analyst system) for an increasingly common manufacturing application involving carbon fiber-reinforced polymer (CFRP) composite and atmospheric pressure plasma treatment.
In the abstract of the paper, it is stated that low inherent surface energy (or the low reactivity of the molecules on the surface of the material) and the “happenstance of surface contamination in multiple manufacturing steps, both contribute to poor adhesive properties in composite materials. Proper surface preparation of pre-bond composite surfaces is. Therefore, an active research topic focused on improving the fidelity of adhesive bonding.”
This active research topic, namely proper surface preparation and cleaning, is our grand obsession. We know that manufacturers have great understanding and control over two of the three necessities for ensuring a great adhesive bond. It’s that third one - knowing that the surface is clean enough and prepared properly before bonding or coating - that manufacturers are still struggling with and that we want to help with.
There is hope. We’ll break down the findings of this study to show how you can protect your adhesion and cleaning process from failure by mastering all three crucial elements for ensuring structural soundness in your products.
Rethink your adhesion manufacturing processes with Surface Intelligence.
Three Basic Elements for Great Adhesion
Excellent adhesion relies on the manufacturer's ability to understand and control three distinct yet interrelated elements. Bonding takes many forms depending on the kind of product being made and what the application is (e.g., painting an aircraft, coating a circuit board, or bonding a composite joint in an automobile chassis), but the fundamental principles of adhesion are always the same. The science behind adhesion is static and reliable.
The three things that must be controlled for adhesion to be successful are:
- The composition of the adhesive, coating, ink, or paint needs to be known and understood to be appropriate for the application at hand.
- The application and curing of the adhesive, coating, ink, or paint need to be managed, so the proper amount of the adhesives or coating is applied and that it is dried or cured at the most effective intervals.
- The quality of the bond surface needs to be understood at the molecular level so that the proper chemical configuration can be achieved during surface preparation and cleaning steps.
All of these present their own challenges as far as control goes, but the first two have been well understood and defined by the suppliers of the adhesives, paints, coatings, dispensing equipment, and curing mechanisms.
The third element is influenced by the widest array of variables and is prone to change without detection as the manufacturing process grinds along. It’s this third element that the writers of this paper were trying to get to the bottom of and understand more fully.
To learn more about how build a more predictable and reliable adhesion process from the ground up (or to optimize an existing process), download our eBook: Predictable Adhesion in Manufacturing Through Process Verification
In their study, they used composite samples manufactured by Hexcel and provided by NASA. Section 2.1 addresses the particulars of just how meticulously the material was produced and handled (like wrapping the test coupons in aluminum foil between each procedure to ensure the surface wasn’t inadvertently changing). This section also outlines the exact film adhesive used and mentions that the bonded assemblies were “cured in an autoclave following the Metlbond manufacturer’s recommended cure cycle.” They were able to rely on the fact that this curing process and the composition of the adhesive itself would have been heavily vetted and tested for optimum effectiveness. Thus, satisfying the first two of the three requirements for controlling adhesion as laid out in our list above.
And yes, even though in the context of this study, those were controlled variables, it evinces how most manufacturers approach their own adhesion process. Manufacturers can reliably fall back on these variables as constants in the adhesion process by following specific curing protocol, assuming all of their equipment is working properly, the resin was mixed correctly, etc. They can readily name the particulars of adhesives and coatings used and can run down the precise needs of the curing process, but when pressed about how ready the surface of their material is for bonding, they typically stop at listing cleaning steps but cannot confidently say that these methods are effective. Mostly they lack confidence because adhesion failures still occur, and the testing programs they use don’t provide actionable data they can use to optimize their cleaning and treatment procedures - which is the third element to control to ensure adhesion.
What the Study Says
The details of the study, in a nutshell, involve the composite material (the CFRP) being manually wiped with an alcohol wipe, plasma treated at varying linear velocities (which is a technical way of saying various speeds), and with three variations on how much each pass of the plasma jet nozzle overlaps the previous passes, and then bonded, cured and strength-tested.
To understand precisely what was happening to the surface of the CFRP at each step, the scientists used X-ray Photoelectron Spectroscopy (XPS) to characterize exactly what kinds of molecules are present and how that composition of particular molecules changes throughout the process. This analysis is necessary because, for adhesion to work, the surface needs to be what we like to call “chemically clean” and chemically reactive. This means the chemicals on the surface of the material being bonded or coated need to be attractive to the chemicals in the adhesive or coating. Adhesion is a chemical reaction that happens at the top 1-5 molecular layers of a surface.
We are unable to show you the exact charts and tables from the study, but below is a table showing the results of a similar test we did at Brighton Science comparing various chemical compositions of surfaces after an isopropyl alcohol wipe, light plasma treatment, and heavy plasma treatment. These data correlate to Table 3 in the study and show that the percentage of the overall molecules on the surface of the CFRP are elements like carbon, oxygen, silicon and others. The data in this table was acquired using XPS to get a clear understanding of what the chemical recipe should be for excellent adhesion and correlate that back to a particular cleaning and treatment regimen.
.png?width=827&name=table-3-chemical-compositions-surface-treatment-light-heavy-plasma-treatment-blog%20(Updated).png)
To determine what processes were creating the strongest bonds, the researchers used a double cantilever beam (DCB) test to break the joints. This test uses equal and opposing forces pulling in opposite directions on the joint assembly, looking at what point the bond doesn’t hold anymore and seeing what part of the assembly (the bulk material, the adhesive itself, or the interface between the two) broke. A strong bond will break within the adhesive or the material itself, and the separated materials will show a somewhat equal amount of adhesive or material left on their surface. To get a very clear illustration of how this works, watch this video of an Oreo cookie being broken (and promptly eaten).
Part of being able to correlate cleaning and treatment processes to bond strength requires a test that can be done in an actual production environment. It should go without saying that it isn’t feasible for manufacturers to use XPS to characterize every surface and then use a DCB test on the finished joints. It’s expensive to do so, and if you’re breaking all your joints, you won’t have any actual finished products. So, it’s necessary to have a test that can be done on the production line, on the actual parts being bonded, that accurately predicts how strong the adhesion will be because the test is sensitive to the chemical nuances present at the molecular level of the surface.
The researchers in this study used Brighton Science's Surface Analyst to conduct a water contact angle measurement test to find this kind of predictability. Water contact angle measurements are done by carefully placing a drop of water on a surface and measuring to what extent that drop spreads out on the surface or beads up, much the way water reacts to waterproof outerwear or newly waxed cars. The angle that the edge of the water makes with the surface (the more the drop beads up, the higher the angle) is what we call the water contact angle (WCA). Often in science, the titles for tests and phenomena don’t make much sense, but thankfully WCA has the majority of information about what is going on right there in the name: water comes in contact with a surface and makes an angle. Water is sensitive to the chemical makeup of surfaces and reacts differently based on how many particular chemicals are present. Consequently, these different reactions tell us much about whether the surface has been chemically cleaned properly for particular adhesion processes.
The Surface Analyst products are uniquely engineered to rapidly, repeatedly, accurately, and easily take contact angle measurements on surfaces of nearly every geometry and every size. The drop of highly purified HPLC-grade water is shot onto the surface using a patented Ballistic Deposition method, which allows for control over the volume and placement of the drop. The measurement of the angle is determined by a top-down vision system in the inspection head which allows for 64 individual measurements to be taken, guaranteeing the accuracy of the angle measured.
There is a bar graph in the study that shows WCAs on the untouched (not yet cleaned or treated), wiped and plasma-treated samples and compares the data from plasma treatments at various speeds (see Table 2). Similarly, Brighton Science has studied the effects of atmospheric plasma functionalization of another commonly used material, PEEK (polyether ether ketone) thermoplastic. Below is a graph that shows how contact angles change (decrease) as the amount of reactive oxygen species increases on the surface by means of plasma treatment.
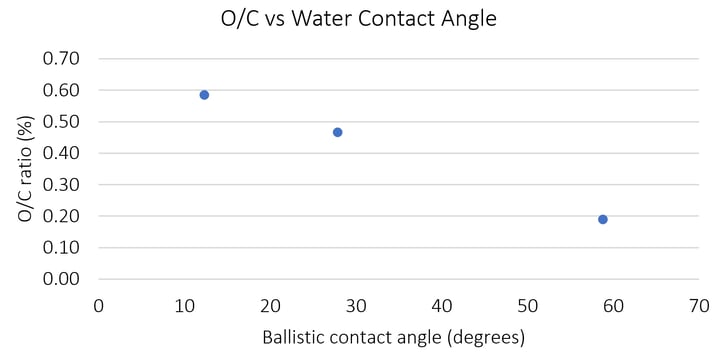
In Table 2 of their study, it’s clear that the WCA is affected dramatically by the plasma treatment each time. The WCA differs somewhat based on speed and overlap, but nonetheless, the water drop creates a much smaller angle on plasma-treated surfaces. Plasma treatment alters the ratio of O/C on the surface so the graph above illustrates how a properly plasma-treated surface results in a low contact angle. In the study itself, you can find that through the XPS analysis and the DCB testing, the changes in WCA correlate beautifully with the optimal chemical composition of the CFRP surface needed to achieve the desired bond strength.
Optimize the power of next-gen connectivity with data & surface intelligence.
At Brighton Science, we use all of these techniques - XPS chemical analysis, DCB bond strength testing, various cleaning, abrading, and treatment methods (such as atmospheric and vacuum plasma treating, grit blasting, solvent cleaning, etc.), and WCA measurements - in our laboratory to help companies better understand and control their adhesion processes. The evidence and conclusions of this study bolster the fact that, in order to have a coating, bonding, painting, sealing, printing, and cleaning process that predictably cranks out the highest quality products, manufacturers need to optimize their cleaning and surface preparation procedures and use production-level testing that measures chemical cleanliness to do so. Helping manufacturers of all industries solve complex adhesion and cleaning problems is what we do every day.
The Surface Analyst products are uniquely engineered to rapidly, repeatedly, accurately, and easily analyze contact angle measurements on surfaces of nearly every geometry and every size. The drop of highly purified HPLC-grade water is shot onto the surface using a patented Ballistic Deposition method, which allows for control over the volume and placement of the drop. The measurement of the angle is determined by a top-down vision system in the inspection head which allows for 64 individual measurements to be taken, guaranteeing the accuracy of the angle measured.
To learn how to build a more predictable and reliable adhesion process from the ground up (or to optimize an existing process), download our eBook: Predictable Adhesion in Manufacturing Through Process Verification


