Lately, the whole world has seen variations of the same conversation. Whether the discussion centers on N95 masks, toilet paper, hand sanitizer, disinfecting wipes, or what movies to watch while maintaining a safe social distance, the underlying concern is about cleanliness.
At Brighton Science, we are completely consumed with the pursuit of cleanliness for strong adhesion to create the most effective manufacturing processes possible. We’ve talked a lot about what cleanliness is and isn’t and how to control cleaning processes in manufacturing to make sure surfaces are bondable. This has raised some interesting questions internally about the nature of the “chemically” clean surfaces that Brighton Sciences' experts specialize in.
So as the minds of the world turn to all things hygienic, we wanted to talk a little about what cleanliness means to manufacturers and how that contrasts with the kind of cleanliness most people are currently concerned about.
What is Clean?
First of all, it needs to be stated that clean is not always clean. It all truly depends on what the purpose of your cleaning is.
As a child, my mother used to toss a washcloth dampened with hot water onto the table after dinner and asked me to clean the tabletop. I’d wipe all the crumbs off, and that was that - clean!
Sometimes when we’re cleaning around the house or in public places, removing large debris by vacuuming a carpet, sweeping a hardwood floor, or removing crumbs from a kitchen table is all we need to do to feel like the cleaning job is done. In order to clean to make sure that illness is not spread around, merely removing dust and dirt isn’t enough.
Similarly, sometimes in manufacturing, particulate removal (like removing the crumbs from the kitchen table) is the biggest concern. This is true of automotive powertrain manufacturing. But if the production process involves any kind of bonding, sealing, coating, painting, printing, or other types of adhesion, particulate removal may not make the material surfaces clean enough. Some industries, like medical device manufacturing, are concerned with cleanliness for adhesion as well as sterilization. That’s why PPE (personal protective equipment) like masks and gloves are critical to maintaining manufacturing environments that are free of every kind of contamination.
That’s where the concepts of chemical cleanliness and biological cleanliness come in.
Rethink your adhesion manufacturing processes with Surface Intelligence.
A biologically clean surface is one that is free of pathogens and viral microbes. This is distinct from chemical cleanliness in that a surface that has been cleaned properly for excellent adhesion may not necessarily be biologically clean. When cleaning a surface to sterilize it, a disinfecting cleaner may be used to cover the surface with an antibacterial liquid (for instance, one containing bleach) and let it sit there to kill the microbes that may be present on the surface.
It is possible to wipe away bacteria and viral activity from a surface (much like what we do when we wash our hands - removing germs from our skin through friction and a surfactant, then sending them down the drain). But the typical surface cleaning done in manufacturing does not create a bacteria-free surface.
Cleanliness for structural bonding in manufacturing requires the removal of the chemical contamination that builds up on a freshly cleaned surface. This kind of cleaning and adhesion is becoming more and more prevalent in manufacturing across all kinds of industries, such as aerospace, automotive, consumer goods, electronics, and packaging.
In order to properly clean a surface for optimal, strong adhesion, there must be a very specific chemical composition created during surface preparation processes. The top 2-3 molecular layers are the “surface” that is being bonded or coated in these adhesion processes.
Look at the image below to get a better sense of what happens to the top few molecular layers of a material surface:
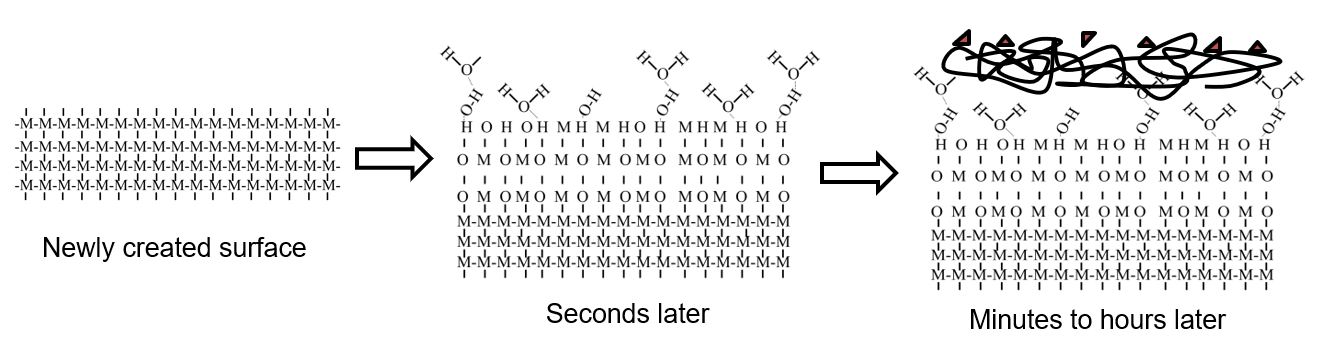
The far left shows a newly created surface where the “M’s” represent molecules of the material itself; let’s say it’s aluminum in this case. The little lines connecting the M’s represent chemical bonds holding the structure together. As you can see, the lines on the top layer of molecules aren’t connected to anything and are eager to bond (or the molecules are highly reactive). We call this surface a high-energy surface.
Moving on to the center, you can see what happens when a surface with high energy sits around for mere moments. The O’s represent oxygen molecules that have attached to the top couple layers of aluminum and oxidized them. This oxidized surface also attracts water molecules in the air, and the whole thing keeps building up until you get to the far right. The far right depicts a surface that is basically impossible to bond to because of carbon-based molecular contamination that has attached to the surface, which will never reliably attach to adhesives, epoxies, coatings, paints, or essentially anything you want to put on that surface.
To give you an idea of how delicate and vulnerable chemically clean surfaces can be - consider a human fingerprint. A typical human fingertip, when lightly placed on a high-energy surface, can deposit a layer of oily contaminants 1,000 layers thick, utterly obliterating the bondable surface. And we all know that it is highly unlikely that if you’re touching a surface, you’re just daintily touching it with one finger. Unintentional human interaction is just one of many dangers to surfaces in manufacturing settings.
Different Purpose, Different Kinds of Clean
Think about the different ways you wipe a surface for different cleaning purposes.
The best practice for chemically cleaning a surface is to use gloves and wipe once in a unidirectional motion across the entire area. Then fold the wipe so an unused side is against the material and drag it across the same surface in the same direction, again, only once. Apply uniform force and uniform speed as you wipe.
When cleaning to disinfect, we use soap and antibacterial liquid (like we stated earlier) to get the surface good and wet to kill the viruses and bacteria. We usually wipe the surface afterward, but this type of cursory wipe is often done with a less-than-pristine wiper (think a paper towel or a dish towel that is just hanging around nearby) and will leave behind readily measurable amounts of cleaning agent and other chemical contaminants.
A contact angle measurement (a highly reliable chemical cleanliness test) on a ‘clean’ countertop almost always reveals the presence of a lot of surfactants left behind by the wiping process. The surface is fine for food preparation in that the microbial count may be very low. This is a great way to clean household countertops, doorknobs, and other commonly touched surfaces, but it does not help adhesion for manufacturers. Likewise, cleaning to get the best adhesion results does not necessarily create a surface you should eat off of.
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
The most important thing to remember is to clean in the most appropriate manner for what you’re hoping to achieve. If you’re building jets and smartphones, you need to have chemically clean surfaces to bond, seal and coat the whole assembly perfectly. If you’re trying to prevent illness, you should wash your hands for at least 20 seconds many times a day, disinfect surfaces and use hand sanitizer.
If you are, in fact, building jets or smartphones and you want to learn more about how to build an adhesion process that fully considers chemical cleanliness, then download our eBook on root cause analysis. This free guide gives you a step-by-step checklist to get to the source of what is causing current adhesion issues and insight into how to prevent these problems when developing new production processes. Download it here: Checklist: Adhesion Failure Root-Cause Analysis for Manufacturers.

