The biomedical industry runs on testing. Testing is at the heart of meeting extensive requirements to ensure healthcare practices and facilities are safe. A kind of testing that has been on all of our minds lately is the kind of testing that analyzes the presence of diseases. Often these tests require a sample of a bodily fluid taken from a patient, be it blood from a fingertip or phlegm from a nostril. Then these samples are often sent off for testing. Some of the unsung heroes in these scenarios are the glass and plastic slides and culture wells upon which these tests are fulfilled.
For manufacturers who build this diagnostic equipment, there’s a great need to test and verify several aspects of their production process and the products going through the process. Testing equipment like diagnostic slides, microplates, silicon wafers, and the still-emerging lab-on-a-chip devices are cut, cleaned, and coated during their assembly, and in order to be effective assays for the end-users, testing needs to be done each step of the way.
Cost and time reduction through high-precision, data-driven verification techniques can give crucial insight to manufacturers who need to have provable uniformity of coatings on the diagnostic equipment they’re building. Many of these devices have incredibly small channels and wells that are designed to guide fluids in a particular manner. If the fluids do not spread, flow or wet out properly on the surfaces of these tools, then they simply aren’t useful as testing equipment.
A great example of this is the lateral flow assays (LFA), like the enzyme-linked immunosorbent assays (ELISA) used to test for COVID-19. The fluid placed on the surface needs to flow uninhibited from one end of the slide to the other.
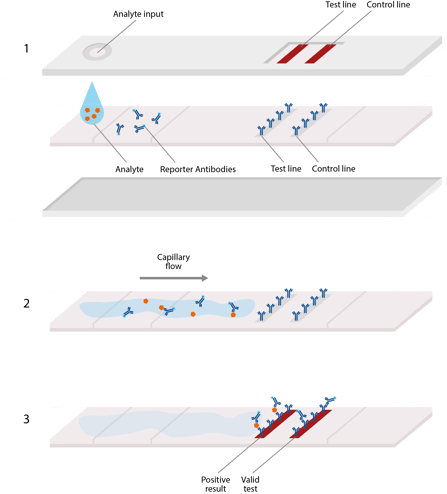
Image via The Native Antigen Company
As the fluid flows over the surface, it comes in contact with various absorbent materials that interact with the fluid and indicate various facts. The fluid flows at the proper rate of its own accord due to a phenomenon known as capillary flow. Capillary flow occurs when the two chemically clean surfaces are close together, and a liquid moves in the cavity between the surfaces, filling in the space. The liquid needs to be attracted to the clean surfaces on a molecular level so it will keep stretching and pulling itself along the surface and reach all the areas of the diagnostic slide it needs to. To achieve this, the surfaces of the glass or polymer slides need to be chemically attracted to the fluid through cleaning and treatment processes.
Rethink your adhesion manufacturing processes with Surface Intelligence.
Whatever the end use of the diagnostics that manufacturers are producing, they need to function properly. They will only work the way they are intended to if the surface treatments are controlled through consistent, data-driven surface quality measurements at each Critical Control Point, or any place the surface quality has an opportunity to change.
Control Your Vacuum Deposition Coating Process
Vacuum deposition is the most common method of applying bio-inert, hydrophilic coatings to diagnostic plates and other devices. Polypropylene microplates can have anywhere from a few hundred to thousands of tiny wells that hold only several microliters of liquids. In order to deposit coatings on the incredibly hard-to-reach surfaces inside each well, a system that completely encapsulates the entire part is required.
There are a couple of vacuum deposition methods that manufacturers generally employ. Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) are broad names for a variety of coating techniques that are used across many industries to apply incredibly thin coatings on surfaces.
One of the most important things to understand about these coating processes is that they are depositing a coating that could be only a few molecules thick.
One of the most common problems for manufacturers of diagnostic equipment is the lack of cost-effective ways to test the uniformity of these coatings. Many companies rely on very expensive and time-consuming spectroscopy techniques to examine the surface before coating and then do a series of performance tests after the coating application. This analysis can tell you a lot about your treatment process prior to coating and how well the coating interacts with fluids (i.e. does the fluid move across the surface in a predictable manner, is the coating introducing bio-contaminants to the samples, etc.). The big drawback is the complexity of these tests and processes.
These coatings are often applied in batches in a chamber where a vacuum is pulled, so being able to do group testing of the coatings on these surfaces fits a typical production model for many companies.
A simple single-fluid contact angle measurement is able to accomplish this verification. With the right inspection equipment, manufacturers can probe inside the extremely small wells of microplates or in the very narrow channels of diagnostic slides with a single drop of bio-inert liquid. One of the factors that make contact angle measurements such compatible tests for these coatings is the sensitivity of the liquid to the molecular differences of surfaces. The liquid drop that is placed on the surface during the inspection will bead up or wet out according to the chemical composition on the surface.
Taking several contact angle measurements on a coated surface will reveal any inconsistencies in the application of the coating. If only a portion of the wells in a plate are properly coated, the entire tool is rejected. Since the parts are prepared in batches, contact angle measurement equipment that provides averages across an entire batch allows manufacturers to quickly isolate issues. For instance, due to cost considerations, new polymers may be used in the formation of these devices, and the chemical composition of those polymers can have an effect on the vacuum deposition process. Being able to immediately spot trends and pinpoint the variable that is perhaps responsible for changes in outcomes can make process validation quicker and easier than lengthy performance tests downstream.
Taking several contact angle measurements can help you map out the surface of a glass slide to ensure the uniformity of a hydrophilic coating. A Materials Science Expert can help you understand how to correlate spectroscopy data that you may already have to a simple contact angle measurement that can reduce the complexity of your testing process.
How to Control Your Plasma Treatment Process
Much like the batch vacuum deposition method of applying the microscopic coatings, the treatment necessary to create a chemically compatible surface is typically done with a vacuum plasma treatment process.
This technique is used for similar reasons to the vacuum deposition process. The ionized gas that is introduced to the vacuum chamber is able to reach areas of the surface that otherwise might be missed by less precise treatments.
In order to use plasma most effectively, you need to know exactly what your surface looks like going into the treatment and what it looks like coming out: heavily contaminated surfaces should be pre-cleaned by detergents or solvents prior to plasma cleaning. When you cut glass slides from a larger piece, wash, and rinse them, you need to be sure none of those steps interfere with the final coating operation.
The contact angle technique recommended for evaluating the coating on a slide or plate can also reveal valuable information about the treatment level prior to coating. A contact angle measurement can flag the presence of contaminants or an under-treated surface so the issue can be addressed quickly and directly.
Contact angle measurements can also be used to test the cleanliness of your vacuum chambers to ensure a long and productive life of your equipment.
To get more information about how to maintain a powerful vacuum plasma system, check out this other blog post.
Using Contact Angle Measurements During Production to Verify Products
The best way to verify each product as it goes through the entirety of the manufacturing process is to utilize contact angle measurements throughout production. Contact angle measurements are not new to medical device manufacturing, but it has typically been done prior to production as a process development tool in a research lab. Harnessing that data in real-time during the cleaning, treatment, and coating processes opens up the possibility of shortening R&D time and creating a common language between development and production.
It’s fast. It’s accurate. It’s non-destructive. It’s the Automated Surface Analyst.
This can be done by building contact angle measurement stations with an inspection device mounted to a fixture where a wafer or slide can be tested quickly and efficiently. Additionally, for microplates with extremely small wells, an automated inspection solution is able to deposit a drop of liquid small enough to take a contact angle measurement even in the most difficult-to-reach places. This ability is a brand-new development and makes in-line contact angle measurements on culture well surfaces possible for the first time ever. Coupling this huge step forward with easily aggregated data on coatings and surface treatments across batches is creating new process control solutions for diagnostic equipment manufacturers.
To learn more about the best ways to utilize water contact angle measurements in your process, download our eBook about verification techniques. The information in this book will put you on the right path to a predictable coating process that results in the highest quality diagnostic products. Download it now: Predictable Adhesion in Manufacturing Through Process Verification