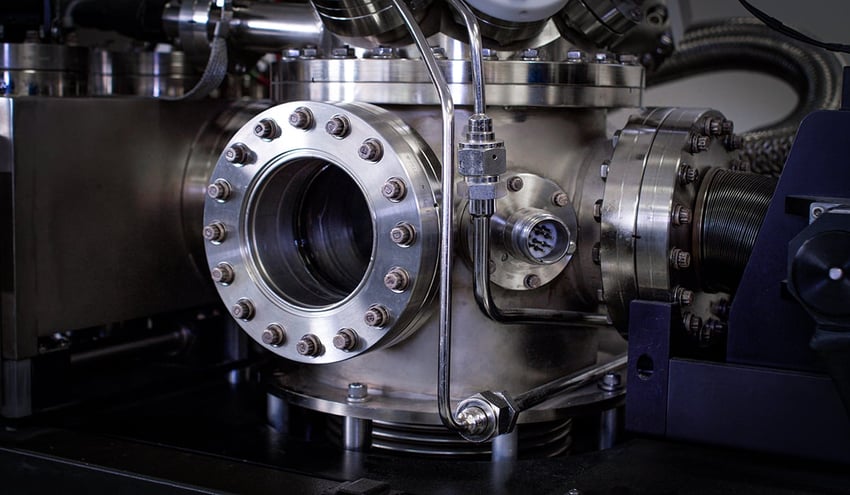
X-ray photoelectron spectroscopy (XPS) is a sensitive surface analysis technique with broad applications across numerous industries. Beyond its fundamental role in materials characterization, XPS can provide critical information to solve real-world material challenges.
With the help of Rose Roberts, Ph.D., Senior Custom Applications and Materials Engineer at Brighton Science, this guide explores what an XPS is, how it works, plus, specific examples illustrating how XPS can address common materials science challenges found in product development and manufacturing, such as contaminant detection, materials characterization, and adhesion enhancement.
What is an X-ray Photoelectron Spectrometer (XPS)?
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive analytical technique used to measure a material surface's elemental composition, chemical state, and electronic state. XPS provides detailed information about the top 1 to 5 molecular layers of a surface, the region essential for ensuring high-quality adhesion processes such as bonding, coating, sealing, painting, and more. This makes XPS invaluable in various fields, including materials science, electronics, and surface engineering.
Watch the video as Rose Roberts, Ph.D. describes what the XPS is in more detail:
How Does X-ray Photoelectron Spectroscopy Work?
XPS operates in an ultra-high vacuum environment, similar to the vacuum of space. The process involves several key steps:
- X-ray Interaction: X-ray beams are directed at a sample surface, causing core electrons to be ejected.
- Electron Ejection: The interaction between X-rays and surface atoms results in the ejection of core electrons from the top 5-10 nm of the substrate surface.
- Energy Measurement: The XPS instrument measures the kinetic energy of the ejected electrons.
- Binding Energy Calculation: The binding energy of the electrons is calculated based on the energy of the incoming X-rays and the kinetic energy of the ejected electrons. Each element has a unique binding energy, enabling precise identification of the elements present on the surface.
This process provides a comprehensive profile of the surface chemistry, revealing the elements present and their chemical states and quantities.
Watch as Rose explains in full detail how exactly the XPS works:
Applications and Benefits of XPS Testing
XPS analysis is widely used due to its sensitivity and precision. Key applications include:
Contamination Analysis
XPS effectively determines quantitative amounts of contamination on surfaces, ensuring clean surfaces in manufacturing processes where even minor contaminant residues can negatively impact adhesion and product performance.
Surface Chemistry Details
XPS can identify slight differences in surface chemistries due to shifts in binding energies. These shifts occur when strong chemical bonds are formed between atoms, providing insights into the chemical states of elements.
Material Analysis
XPS can provide information about the chemical state of elements, including the difference between various chemical environments of silicon, such as elemental silicon (as in a wafer), silicon dioxide (silica), or silicon bonded to organic groups (as in a polymer). This information is vital for understanding the material's bonding capabilities and suitability for different applications.
Adhesion Studies
By measuring the composition of the top few molecular layers, users gain an understanding of surface chemistry and how well adhesives may bond to that surface. This understanding is crucial for developing strong adhesive bonds.
In the next video, Rose explores in-depth what an XPS can be used for:
Who Can Operate an XPS?
Now that we know what an XPS is, what it’s used for, and how it works, the next question is - who exactly can operate this instrument?
In the following video, Rose breaks down the expertise necessary to operate this instrument:
Case Studies and Practical Examples
Detecting Contamination
Ensuring surfaces are clean is crucial for successful quality control in manufacturing. XPS detects and quantifies surface contaminants that can cause problems within manufacturing. For example, in electronics, XPS can identify trace contamination that could disrupt soldering processes in electronic manufacturing, leading to unreliable connections and ultimately, failure.
Figure 1. After cleaning a metal surface with different detergents, XPS was used to determine the surface cleanliness of the metal. Carbon content is characteristic of a hydrocarbon oil, so a surface that contains more oil will exhibit a higher carbon/metal (C/M) ratio. Detergents E, F, and G can much more easily remove this particular oil from the metallic surface, as the C/M ratio is low after cleaning. Water contact angle can also correlate very well with surface state/cleanliness of the material.
Improving Adhesion
Adhesion failure is common in many industries, from automotive to aerospace. By analyzing surface composition and chemistry, XPS helps identify root causes of adhesion failure and guides the development of surface treatments that enhance adhesive bonding performance. For example, XPS data can reveal the presence of surface oxides that hinder adhesion, allowing for targeted surface treatments to improve bond strength.
Summarizing the XPS
XPS systems are indispensable tools for surface analysis, providing detailed insights into material surfaces' elemental composition and chemical states. Their ability to detect and quantify surface contaminants, differentiate chemical states, and analyze specific areas makes them invaluable for ensuring strong adhesive bonds and understanding material properties.
For our final video, Rose summarizes the XPS and how Brighton Science uses this powerful equipment to help manufacturers better understand the chemical composition of their surfaces:
By leveraging the power of XPS, industries can enhance their understanding of surface phenomena, leading to improved product performance and reliability.
Boost product quality and reliability with better adhesion. Get the surface analysis insights you need in our eBook "What is Adhesion?"