This is part one of a two-part series explaining the finer points of Brighton Science's approach to helping companies build reliability into their cleaning and adhesion processes through consultation and implementation of novel inspection equipment. These two articles are based on this technical paper written by Dr. Giles Dillingham about the best way to measure surface quality (hint: you almost always need to use only one liquid and not two or more, but we’ll get into why a little later).
You can go directly to part two if that teaser was just too good to resist.
How Does Adhesion Work?
There are times when we say something to the effect of “leave the science to the experts” so manufacturers don’t have to get bogged down in the details and can just focus on building great products. However, in order to understand what the best methods are for controlling manufacturing processes that are steeped in Chemistry and Materials Science, being able to drill into the minutiae and vet the options available is extremely valuable to companies. Manufacturers do this sort of comparative analysis for all aspects of their processes. So, leaving critical surface quality up to chance permits gaping holes where basic knowledge about the science of surface quality analysis could allow for informed decision-making about optimizing cleaning and adhesion processes.
Rethink your adhesion manufacturing processes with Surface Intelligence.
To get a full picture of how adhesion works and how to study it so you can control and predict it, you need to start at the absolute beginning: Solids and liquids exist because all atoms and molecules attract each other. Even gases with low intermolecular attraction (think extremely light gases like helium or nitrogen) become liquids if they are cooled enough (think liquid nitrogen). The same intermolecular attractive forces that hold a substance together will attract other substances, and these forces are what cause the molecules in a paint or an adhesive to stick to the molecules that comprise a substrate. This is adhesion at its most elemental. The interface where the molecules at the top few molecular layers of a material’s surface create chemical bonds with the molecules at the top few molecular layers of a coating (such as paint), an adhesive (such as epoxy), or another material (such as copper for welding or brazing) is where adhesion happens.
The force of attraction of one molecule for another depends on the structure of the molecule: some structures are strongly attractive, while some are only weakly attractive. Treating a surface through cleaning or oxidizing the surface with a flame, corona, or plasma can cause it to attract a paint or adhesive more strongly. What is happening during these treatments is that molecules on the surface of the material being treated that have weak attractive forces are being replaced with molecules that possess stronger attractive forces.
These types of treatments are extremely common in manufacturing these days when surfaces need to be chemically altered. Oftentimes, these treatments are paired with and are preceded by a different kind of cleaning step to remove larger debris and residues so that the plasma, flame, or corona treatment can actually interact with and change the molecules on the actual surface of the raw material. This is one of the reasons why it’s so important to understand what these treatments are actually doing when they’re used. Sending a part that appears clean but might have some oils on it into a vacuum plasma chamber may not give the results you're looking for. The plasma will interact with the molecules that it can reach, and if there is a residue several molecules thick on that surface, it may be invisible to the human eye, but the plasma will treat the wrong surface, which could lead to weak or unpredictable bonds once a coating or adhesive is applied and that oil is still on the material.
So, this should create a question in our minds about how to know if an invisible residue is on the surfaces of our materials. How can manufacturers be certain that their parts are clean enough to coat or paint or for molten metal to flow freely over the surface and bond strongly to another metal, like in a brazing or soldering operation?
What is Surface Energy, and How Do I Measure it?
One way to characterize the attractiveness of a surface towards other substances is by a property called surface energy: a high-energy surface is generally attractive to other substances; a low-energy surface is not. Examples of high-energy surfaces are clean metals or glasses: Paints and adhesives stick to them strongly. Low-energy surfaces are exemplified by substances like polyethylene or polytetrafluoroethylene (PTFE): paints, adhesives, foods, and most other things are not attracted to these surfaces and do not stick. Polymers, like the two we just mentioned, are frequently formulated with the intent to have a lubricious, hydrophobic (liquid-repellant), and generally non-sticky surface. These innate properties serve various purposes, but in order to assemble full, usable products, we need to be able to bond these substances to other materials or be able to coat, paint, and print on them.
All liquids and solids have surface energy. The surface energy of a liquid is usually referred to as its surface tension, but surface tension and surface energy are the same things. This principle is crucial for understanding how to measure the surface energy of a surface you want something to adhere to.
Considering that the ability of the surface to attract or repel another substance is the fundamental property of interest, a direct measurement of this ability is the ideal control metric for predicting adhesion outcomes or cleanliness level. This concept is the basis of wetting measurements using dyne inks or contact angle-based methods: both are techniques for probing how strongly a surface attracts a liquid. Wetting or wettability refers to the behavior of the liquid on the surface and how much it spreads out, uninhibited, or how much it beads up, constricts, or breaks up into droplets when it comes into contact with the material’s surface. Both methods are commonly used in many industries, although they differ greatly in their accuracy and precision.
Controlling Surface Properties Through Surface Energy Measurement
Controlling a cleaning process or surface treatment process requires a way to measure the surface characteristics that are affected by these processes. We should stop here a moment and emphasize that we should be most interested in the characteristics that are actually affected by cleaning and treatment processes. There will be much more on that in the second part of this series, but it bears repeating that if we want an accurate accounting of what changes are occurring on surfaces to make them sufficiently prepared for successful downstream production steps, then we need to be certain that we are evaluating surface quality based on the changes that are actually happening. That seems self-explanatory, but many of the methods available to inspect surface quality are deficient in providing concrete information about the chemical modifications occurring throughout manufacturing processes or giving a quantitative value to these changes so they can be comparatively evaluated and tracked.
Many methods are subjective and have no quantifiable component whatsoever, like a water break test. Many approaches are destructive, like a tape pull test, or are not meant to measure surface energy or the chemical composition of a surface, like ion chromatography, so they aren’t able to be used as a true process control technique when it comes to monitoring adhesion processes.
There are several ways to look at the actual chemical makeup of a surface. We can analyze the molecular composition and structure directly through the use of analytical instruments like an X-ray Photoelectron Spectrometer (XPS) or an infrared spectrometer (FTIR). These techniques are expensive, often relegated to laboratories, and (much like the techniques mentioned above) not practical for use in process control in a manufacturing facility.
Dyne Inks
Dyne inks are the most frequently encountered method of surface energy evaluation used on production lines or in manufacturing in general. Dyne inks consist of a series of liquids having a range of surface tensions (surface energies) generally between 30 and 72 dynes/cm. Dynes/cm is the unit of measurement for surface tension (force per unit length) and is often shortened to just a “dyne” number. The various surface tensions in the inks are created by mixing two or more mutually soluble liquids of different surface tensions in different proportions. Commercially available mixtures include ethanol and water or formamide and 2-ethoxyethanol. Usually, a blue or purple dye is added to the mixture to improve visibility on the surface.
In use, a thin film of the liquid mixture is spread on the surface to be tested; if the liquid film breaks up into droplets in less than two seconds, the test is repeated with a mixture of lower surface tension. The surface tension of the liquid mixture that will stay spread in a continuous film on the surface for at least two seconds is defined as being equal to the “wetting tension” of the surface. This “wetting tension” number (30 to 72) is used as an approximation of the surface energy of the surface under evaluation but suffers from low accuracy and precision as well as poor reproducibility. Furthermore, because dyne inks are solvents, they clean the surface of contaminants during measurement and, therefore, frequently do not detect them. These inks are designed only to be used on polymers and, if used on metals, will almost always give an inaccurate result.
Contact Angle
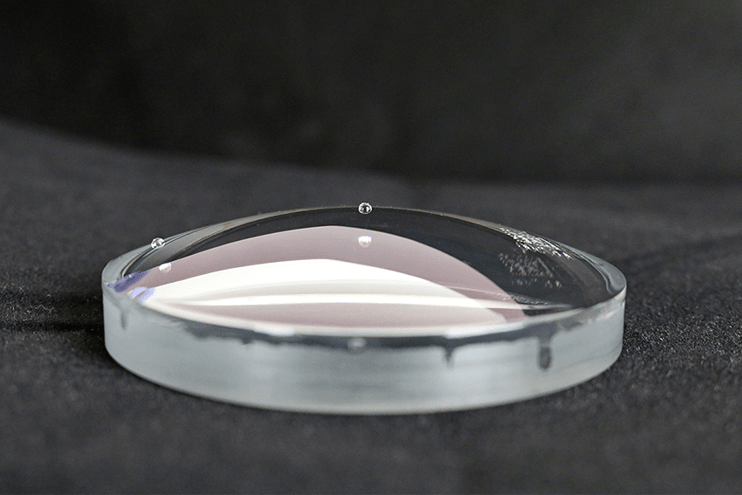
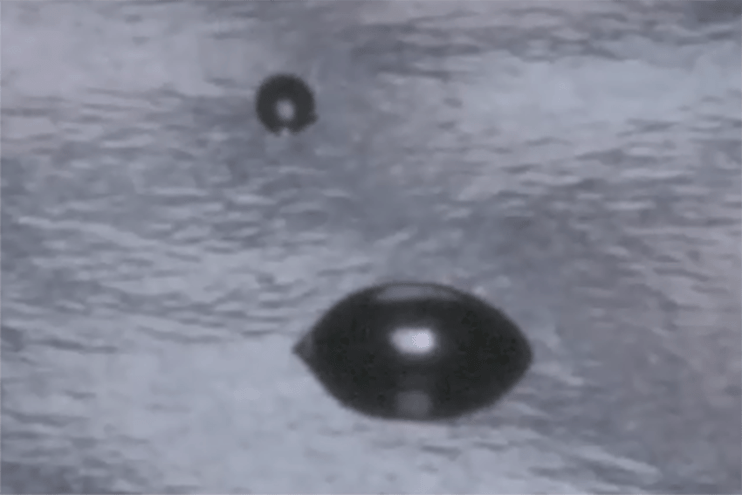
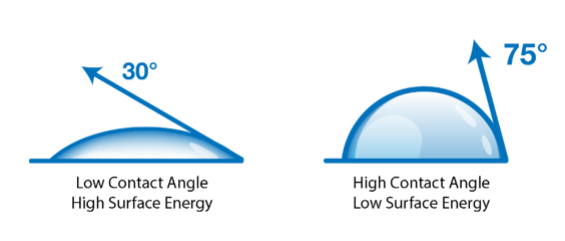
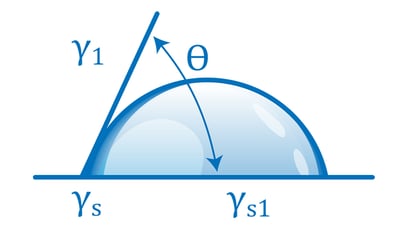 Contact angle-based methods are much more quantitative and much less subjective than dyne ink methods. The two surface quality evaluation methods are similar in that they both look at how a liquid behaves on the surface of a material as the liquid wets out or beads up. Contact angle methods involve measuring how a drop of liquid placed on the surface spreads, usually by measuring the angle formed by the perimeter of the drop and the surface. A liquid drop that spreads a lot has a small contact angle; this means it is attracted to the surface more strongly than it is attracted to itself. A drop that spreads very little (‘beads up’) has a large contact angle: its molecules are being attracted more strongly to themselves than to the surface. The contact angle of the liquid on the surface changes with the chemical state of the surface in a very predictable and reproducible way.
Contact angle-based methods are much more quantitative and much less subjective than dyne ink methods. The two surface quality evaluation methods are similar in that they both look at how a liquid behaves on the surface of a material as the liquid wets out or beads up. Contact angle methods involve measuring how a drop of liquid placed on the surface spreads, usually by measuring the angle formed by the perimeter of the drop and the surface. A liquid drop that spreads a lot has a small contact angle; this means it is attracted to the surface more strongly than it is attracted to itself. A drop that spreads very little (‘beads up’) has a large contact angle: its molecules are being attracted more strongly to themselves than to the surface. The contact angle of the liquid on the surface changes with the chemical state of the surface in a very predictable and reproducible way.
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
For example, clean metals, ceramics, and glasses establish low contact angles. Contamination of these surfaces causes the contact angle to increase: the liquid is not attracted strongly to the contaminant. Untreated polymers have high contact angles; oxidizing the surface of a polymer by corona, flame, or plasma causes the contact angles to decrease because the oxidized surface attracts the liquid more strongly. The contact angles change up or down in a smoothly predictable way depending on the level of contamination or the treatment level. This is why they provide an excellent metric for process control.
To learn more about contact angle and how to implement it in your manufacturing operations, download the eBook "What is Contact Angle?"