Key Takeaways
- Surface free energy and surface energy are interchangeable terms in manufacturing contexts; both describe the energy available at a material’s surface to enable interactions such as bonding, coating, printing, and cleaning.
- Surface energy has two components, dispersive and polar. Only the polar component can be intentionally engineered through treatments and cleaning.
- Measuring a material’s polar component via water contact angle (WCA) provides a precise, repeatable proxy for surface energy and directly correlates with adhesion performance.
- Manufacturers use surface energy measurement to control Critical Control Points (CCPs) in bonding, coating, and cleaning workflows.
- Integrating contact angle data into BConnectSM enables in-process traceability, process development, quality control, and supplier verification.
Understanding Surface Free Energy vs. Surface Energy
In practical manufacturing language, surface free energy and surface energy mean the same thing: the energy available at the surface of a material to do work. “Free energy” is the more formal thermodynamic term, but engineers and scientists typically shorten it to surface energy because the domain of interest is always the material’s surface.Surface energy determines how surfaces interact with adhesives, coatings, inks, cleaning agents, and other materials. Yet despite its importance, surface energy often goes unmeasured, and therefore uncontrolled, resulting in unpredictable failure modes in bonding, coating, and assembly.
Controlling surface energy requires two capabilities:
- Altering the surface (via cleaning, plasma, flame treatment, etc.)
- Measuring the surface at each step to verify when chemical changes occur
Rethink your adhesion manufacturing processes with Surface Intelligence.
What Actually Creates Surface Energy?
Surface energy comes from the attraction between molecules at the surface of a material. These attractions arise from the distribution and movement of electrons within those molecules, and they can be grouped into two categories: dispersive and polar forces.
Together, these two components determine a surface’s ability to interact with liquids, adhesives, coatings, and contaminants.
Dispersive vs. Polar Surface Energy
Dispersive Surface Energy
Dispersive forces are created by temporary fluctuations in electron clouds around molecules, producing short-lived positive and negative areas that attract other molecules. These interactions exist in all materials and are relatively weak.
Dispersive forces differ from material to material, but they are not meaningfully adjustable through manufacturing processes. Polystyrene, PVC, and other polymers with aromatic rings or larger electron systems tend to have higher dispersive components, but these values remain essentially fixed.
In short: dispersive forces are real and measurable, but they are not something manufacturers can control.
Tip from a Surface Scientist:
If a force cannot be influenced by cleaning or treatment, it doesn’t belong in your process-control strategy. Dispersive forces fall into that category.
Polar Surface Energy
Polar forces arise from permanent dipoles, which are regions of a molecule that always have slightly positive or slightly negative charge. These interactions are significantly stronger than dispersive forces and are directly responsible for the ability of adhesives, coatings, and inks to form strong bonds with a surface.
Unlike dispersive forces, the polar component of surface energy can be engineered. Processes such as plasma treatment, corona treatment, flame treatment, solvent cleaning, aqueous cleaning, or light abrasion alter how electrons are distributed on a surface, raising or lowering the polar component.
Because polar forces respond to surface conditioning, they are the part of surface energy that manufacturers intentionally control.
Tip from a Surface Scientist:
If you’re adjusting a process to “improve adhesion,” what you’re really doing is modifying the polar component of the surface energy, even if you’re not calling it that.
Why the Polar Component Matters Most
Polar interactions determine whether a surface will wet properly, hold onto an adhesive, or resist contamination during storage. They also degrade fastest when surfaces are not controlled or when cleaned parts sit before use.
Even environmental exposure, such as packaging materials or airborne contaminants, can lower the polar component over time.
This makes ongoing measurement essential for maintaining consistent adhesion and coating outcomes. The most practical way to track these molecular changes is through water contact angle.
How Surface Free Energy Is Calculated
Surface free energy is often expressed as the sum of its two components:γS = γSd + γSp
Where:
- γS = total surface free energy
- γSd = dispersive component
- γSp = polar component
While this formula is conceptually useful, calculating total surface energy directly is complex and susceptible to error. Manufacturers typically avoid full calculations and instead measure the polar component through WCA, which is more stable, sensitive, and proven to correlate with adhesion performance.
When to Use “Surface Free Energy” vs. “Surface Energy”
In real-world manufacturing, there is no functional difference between the two terms. Both refer to the energetic condition of a surface relevant to bonding, printing, coating, and cleaning.
What matters is understanding which part of that energy your process can control, and that is the polar component.
Surface energy is therefore a practical engineering parameter, not just a theoretical concept. It represents the molecular readiness of a surface to participate in adhesion-driving interactions.
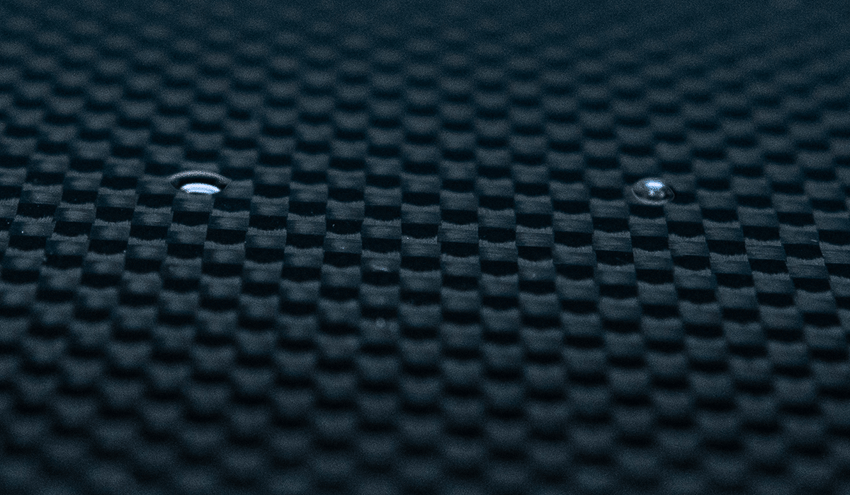
Why Water Contact Angle Is the Best Indicator of Surface Energy
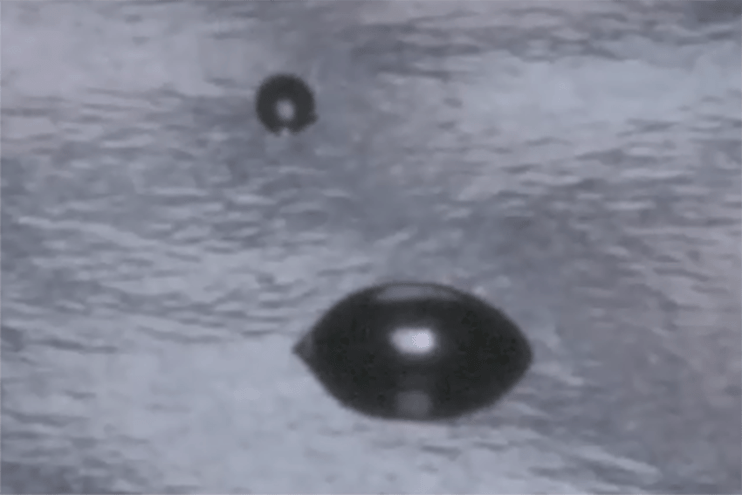
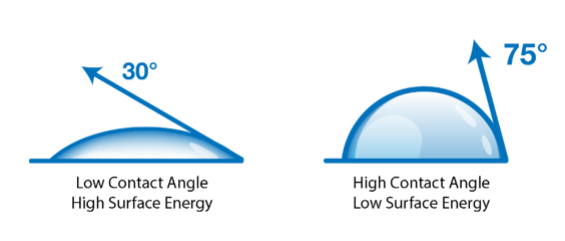
Because water is highly polar, the angle it forms on a surface directly reflects how strongly that surface attracts polar molecules. That makes WCA a reliable, repeatable indicator of changes in surface chemistry, particularly changes caused by treatment or contamination.- A surface with higher surface energy allows water to spread, producing a lower contact angle.
- A surface with lower surface energy causes water to bead, producing a higher contact angle.

This relationship makes WCA the most practical method for monitoring surface readiness throughout production. It’s why tools such as the Surface Analyst and the BConnect platform are built around capturing, tracking, and analyzing contact angle data.
Tip from a Surface Scientist:
If you catch a shift in contact angle early, you prevent downstream defects. If you wait until the bond fails, the surface has been wrong for hours, or days.

Bringing It All Together
Surface energy, whether you call it surface energy or surface free energy, is a critical manufacturing property that determines how well surfaces interact with the materials applied to them. Although both dispersive and polar forces contribute to total surface energy, only the polar component responds to cleaning and treatment. This makes it the primary focus for process development, quality control, and supplier verification.
By incorporating contact angle measurement at key Critical Control Points and integrating that data into BConnect, manufacturers gain real-time visibility into surface quality and the molecular conditions that drive adhesion reliability.
To deepen your understanding of contact angle and its role in manufacturing and supply chain quality, explore the eBook “What Is Contact Angle? Bridging the Gap: How Contact Angle Insights Drive Manufacturing & Supply Chain Innovations.”
Q&A: Fast Answers for Engineers
Q: Are surface free energy and surface energy different?
No. In manufacturing applications, they are used interchangeably.
Q: Which component controls adhesion performance?
The polar component, which changes with treatments and correlates with bond strength.
Q: Can dispersive surface energy be adjusted?
Not realistically. It’s inherent to the material.
Q: Why measure water contact angle?
It reflects changes in the polar component and is highly sensitive to contamination and treatment effectiveness.
Q: Does surface energy degrade over time?
Yes. Clean surfaces can attract airborne molecules or packaging residues that reduce surface energy.
Q: How can manufacturers control surface energy?
By measuring contact angle at critical process points and adjusting cleaning/treatment steps based on that data.