These days, if you see a painted product, it is likely a polymer-based coating providing a striking and powerful barrier between the underlying material and elements in the atmosphere that want to corrode that material. Polymer coating technology has advanced tremendously in the last decade. Coatings are more economical, durable, provide better corrosion resistance, and on top of all that, are less damaging to the environment.
Whether a polymeric coating is applied as a liquid with the polymer dissolved in a solvent or dispersed in a latex, or in a powder form with the polymer ground into fine, dry dust, these coatings are a crucial component to essentially all manufacturing sectors. Our infrastructure (i.e. bridges, oil pipelines, transportation, etc.) relies daily on these coatings doing their job and protecting the metals underneath from corrosion and degradation.
To ensure polymeric coatings provide the necessary protection and also maintain their appearance (since aesthetics is an equally important purpose of paints and coatings), the coating itself must be able to block moisture, and the integrity of its bond to the metal must be precisely controlled during manufacturing.
The fate of every painted vehicle, bridge, appliance, building, and piece of patio furniture rest in the hands of manufacturers who can guarantee the reliability of polymeric coatings by measuring and controlling the cleanliness of the surfaces they are applied to.
What is a Polymer Coating
A polymer coating, at its most fundamental level, is a paint or coating that is made with polymeric binders to provide protection against corrosion and visual appeal. A polymer is a compound molecule that is made up of simpler molecules called monomers in a large chain of molecules, which gives these substances their functional properties as a resistive layer against anything that would try to break down the material underneath, such as moisture, acids, solvents and the like.
A paint, in its most basic form, is simply a polymer dissolved in an appropriate solvent, such as mineral spirits, lacquer thinner, or sometimes water, that is applied by brush, spray, or dip. The solvent evaporates and leaves the polymer behind as a coating. Typically paints contain many functional additives beyond the polymer, including pigments, wetting agents, viscosity modifiers, and more. More recently, techniques have been developed for applying paints without the need for solvents and viscosity modifiers.
Struggling with adhesion failures? Been there. Solved that.
Powder coatings, on the other hand, are ground-up polymers blended with pigments and other additives that are sprayed onto electrostatically charged surfaces. Once coated, the part is heated to melt and fuse the powder particles to the surface. Because the application process doesn’t include liquids, powder coatings can be applied thicker and more economically than liquid paints.
Polymer Coating Advantages
There are significant advantages to polymer coatings over traditional paint processes. Powder coatings are 100% solid coatings that don’t require any solvents. This makes them very attractive to manufacturers because they don’t include the added expense of purchasing or disposing of solvents, and they allow manufacturers to be more compliant with volatile organic compound (VOCs) regulations intended to protect the environment and air quality.
Powder coatings also have the advantage of producing very little waste during the application process. Traditional painting processes try to avoid overspray that results in lost paint--otherwise known as scrapped material. Unlike traditional painting processes, powder coating overspray can be collected and reused.
One of polymer coating's most substantial advantages is the lack of impact they have on other properties of the materials they are applied to. For instance, if a polymeric coating is used to protect a container meant to transport and store chemicals, the coating does nothing to compromise the safety and mechanical strength of the material it coats. Over the last ten years, research has seen many advancements in functional coatings that have increased ease of use, are self-cleaning, antibacterial, and have antifouling properties.
In general, polymer coating processes can use coatings that are much harder, more heat resistant, can adhere better to the substrate, and are much more durable than traditional coatings.
The Polymer Coating Process
Water-based paints don’t include VOCs, overcoming the environmental issue mentioned earlier, but they are much less durable than polymer coatings due to their sensitivity to the surface cleanliness of the materials they are applied to. If a surface isn’t cleaned to an extremely high standard, water-based paints have a tougher time adhering to the surface.
Surface preparation is always a critical process step to ensuring strong adhesion of polymer coatings, but it’s important to prepare surfaces appropriately for the application. The type of coating and the properties of that coating are necessary to understand when developing a surface cleaning and treatment process.
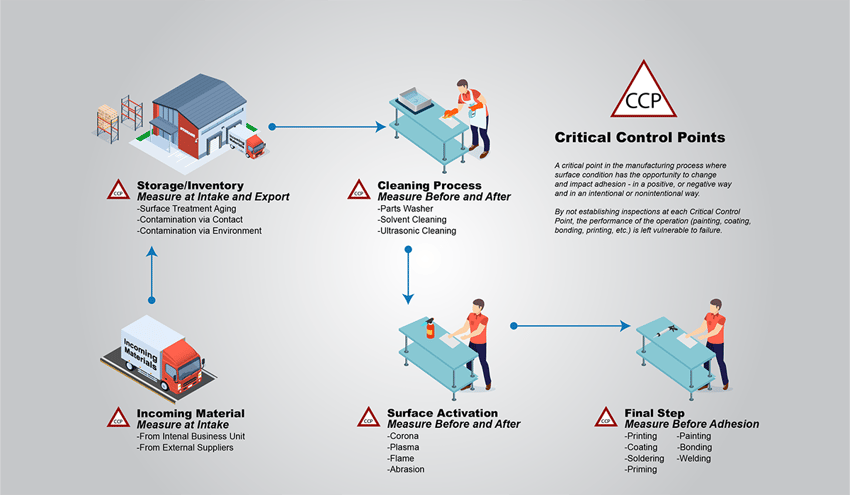
Typically, in manufacturing, the term polymer coating is shorthand for powder coating, even though liquid paints are also polymer films adhered to a material surface. A typical powder coating process begins with an operator loading the parts to be coated onto a conveyor that might be suspended from the ceiling or on the floor. This is the first Critical Control Point (CCP), or any point in the manufacturing process, where a material surface has the opportunity to change chemically (become contaminated or have contamination removed). The introduction of parts into a process is always the first CCP, and manufacturers need to take care to measure the surface cleanliness of all incoming parts before being processed to get a base-level understanding of how soiled or contaminated materials are at the very beginning. This step allows manufacturers to catch critical cleanliness issues before the part gets processed and costs the company time and money with rework and scrap.
The next step, and the second CCP in this process, consists of cleaning procedures using any combination of methods ranging from spray washing, chemical cleaning, etching, and then rinsing and drying. This is the process gap that, if left unchecked, can result in orange peel texturing in the coating, pinholes, inconsistent coverage, and a whole host of other very common failures.
It has to be understood that every time something comes into contact with the part, even if that something is soap, a technician’s hands moving it from one step to the next, or even just water, molecular-level contaminants are able to be transferred to the surface and cause the powder coating to adhere poorly or not at all.
Clean surfaces are very delicate and can change rapidly. This is because clean surfaces have what surface scientists call “high surface free energy.” When a surface is clean, it wants to react with anything it comes into contact with, which is great for adhesion but also means that if a surface is exposed to air, contaminated ‘cleaning’ detergents, or unclean machinery, substances that are adverse to adhesion can transfer to the material surface.
The application of polymer coatings usually involves spraying equipment that covers the surface of suspended parts on an automated assembly line. These parts not only consist of large flat surfaces that are easy to coat, but they also include tight corners, edges, curves, mechanical fasteners, and other tiny areas that pose difficulties to manufacturers looking to apply a consistent and uniform coating. These areas are not only difficult to apply a coating to due to a phenomenon called the Faraday Cage Effect, but they are also more difficult to guarantee the cleanliness of, which can lead to the polymer coating pulling back from those areas. If contaminants that are detrimental to the adhesion of a polymer coating are present in one of these small spaces, it can be extremely difficult to detect them and prevent coating failures.
The process of powder coating can be tricky as there are several new variables at play, like the necessity to ground the metal part being coated and whether the plastic powder has been reclaimed and reused properly, etc. But there is one aspect of the process that is absolutely vital and often overlooked, or at least uncontrolled.
Before a coating can adhere well to a surface, the surface needs to be thoroughly cleaned and prepared. This almost goes without saying. There is no powder coating process that does not require some manner of cleaning and surface preparation; however, these steps are usually only monitored visually, if at all, due to a lack of effective cleanliness measurement tools available for use on the production line.
Whether the powder coating process is done manually on a few parts every hour or is done through automation that is able to coat tens of thousands of parts every day, if there is no way to measure how clean the surfaces are before applying the coating, then failure is inevitable, and costs are high.
Common Polymer Coating Failure Types
Polymer coating failure is a massive economic cost to society. Every major bridge in the United States is rusting, proper maintenance of any metal fire escape requires consistent reapplication of protective coatings, and corrosion is a leading reason for oil pipeline leakage and spills.
 In 2013, 70,000 bridges qualified as “structurally deficient” across the country. That same year, the American Society of Civil Engineers report card for American Infrastructure gave the nation a D+ on its infrastructure, and it put the estimated investment needed by 2020 at $3.6 trillion. The Association for Materials Protection and Performance (AMPP, formerly NACE - National Association of Corrosion Engineers) Institute estimates that “unmitigated corrosion costs the U.S. economy over $500 billion each year, or roughly 3.1 percent of our GDP.”
In 2013, 70,000 bridges qualified as “structurally deficient” across the country. That same year, the American Society of Civil Engineers report card for American Infrastructure gave the nation a D+ on its infrastructure, and it put the estimated investment needed by 2020 at $3.6 trillion. The Association for Materials Protection and Performance (AMPP, formerly NACE - National Association of Corrosion Engineers) Institute estimates that “unmitigated corrosion costs the U.S. economy over $500 billion each year, or roughly 3.1 percent of our GDP.”
According to the United States Cost of Corrosion Study, produced by AMPP, corrosion costs the US oil and gas exploration and production industry $1.4 billion a year.
These issues can be largely mitigated by protective polymeric coatings that ward off corrosion and support structural integrity.
The longevity of a polymer coating and its ability to protect the underlying object from corrosion or degradation depends on two factors:
- The ability of the coating to prevent moisture from getting through
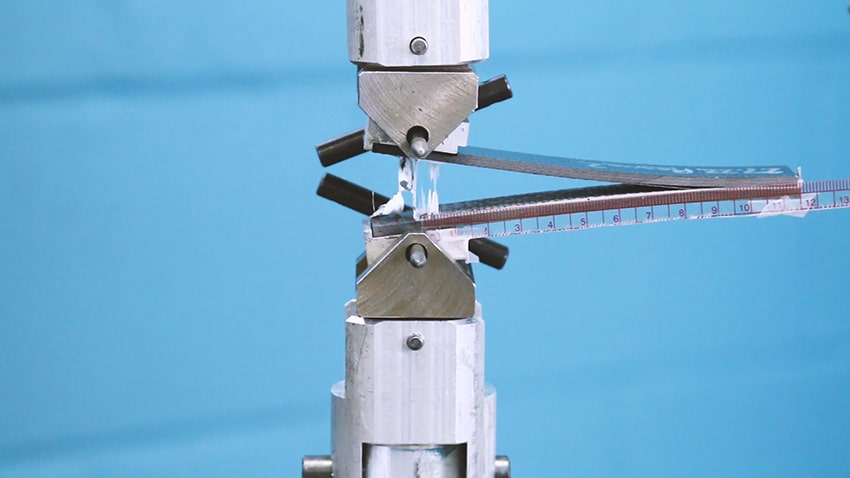
- The quality of its adhesion to the substrate
These two characteristics are closely related because reliable adhesion helps fight against moisture reaching the underlying material.
Coatings will all eventually degrade. The interface between the polymer coating and the substrate is not 100% chemically stable. This is particularly true when the substrate is steel. No material has the combination of strength and low cost of steel, so it is still the material of choice for bridges, pipelines, and cars, but it rusts. Unlike a polymer-steel interface, the interface between a polymer coating and a polymer substrate is much more chemically stable and, therefore, more durable. That’s one reason why automotive manufacturers (and increasingly, civil engineers!) are looking to advanced composite materials for their newest designs: a coated plastic or composite has no moisture-sensitive metal-polymer interface to degrade over time.
When we talk about chemical stability, we’re referring to maintaining integrity in the presence of any moisture that diffuses through a polymer coating to the interface between the polymer and the substrate. It's this moisture that causes corrosion and loss of adhesion.
Intelligent paint formulation reduces the rate of moisture permeation but can never stop it entirely, and good surface treatment techniques reduce the rate of corrosion of the substrate but can never stop it entirely.
However, when both the coating and the surface quality are controlled, the degradation rate can be slowed down to the point where it's essentially imperceptible, and the protective quality of the coating can last for many, many years.
How to Ensure a Strong and Durable Polymer Coating
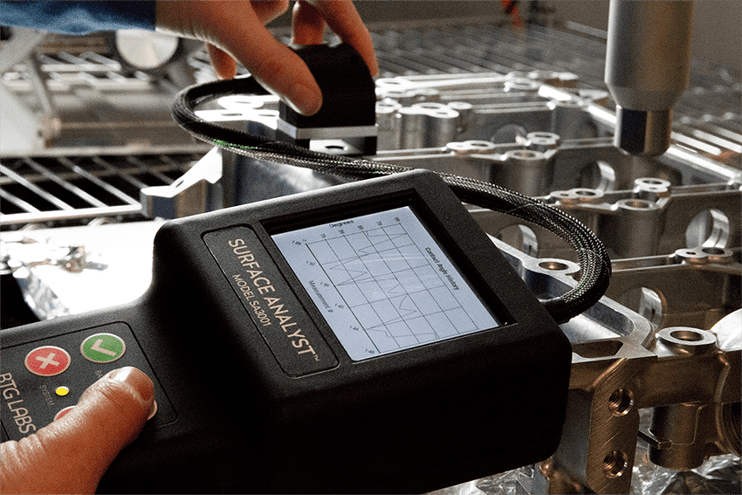
The first step is to ensure the surface to be coated is uniformly clean at a molecular level. Strong, reliable adhesion occurs when two substances are chemically compatible at the top 2-5 molecular layers of their surfaces. Any cleaning, treatment, or preparation process alters the molecular makeup of surfaces. It is essential to measure the cleanliness of surfaces throughout the manufacturing process with a method that is sensitive to these molecular-level changes.
The most convenient and sensitive way to interrogate the cleanliness of a surface is to use rapid contact angle measurements optimized for manufacturing. These quantitative measurements offer a precise process control method so manufacturers can predict coating outcomes and build high-performance products.
Rethink your adhesion manufacturing processes with Surface Intelligence.
Polymer coating technology has advanced tremendously in recent history. Polymeric coatings are more economical and durable, provide better corrosion resistance, and are less damaging to the environment than traditional paints. However, the quality of the polymer coating is only as good as the quality of the substrate surface. Good manufacturing practices that involve measurement and control of surface quality are crucial to manufacturers who want to increase yield, reduce risk and waste, and build reliable products or buildings and bridges.
To learn more about designing polymer coating processes that don’t leave anything up to chance, download our free eBook: The Future of Manufacturing: A Guide to Intelligent Adhesive Bonding Technologies & Methodologies."































.jpg?width=850&height=495&name=metal-brazing-copper-component%20(reduced).jpg)